14+ calculate h3o
Use E for scientific notation. Web The relationship between H3O and OH- in an water is H3O x OH- 10-14.
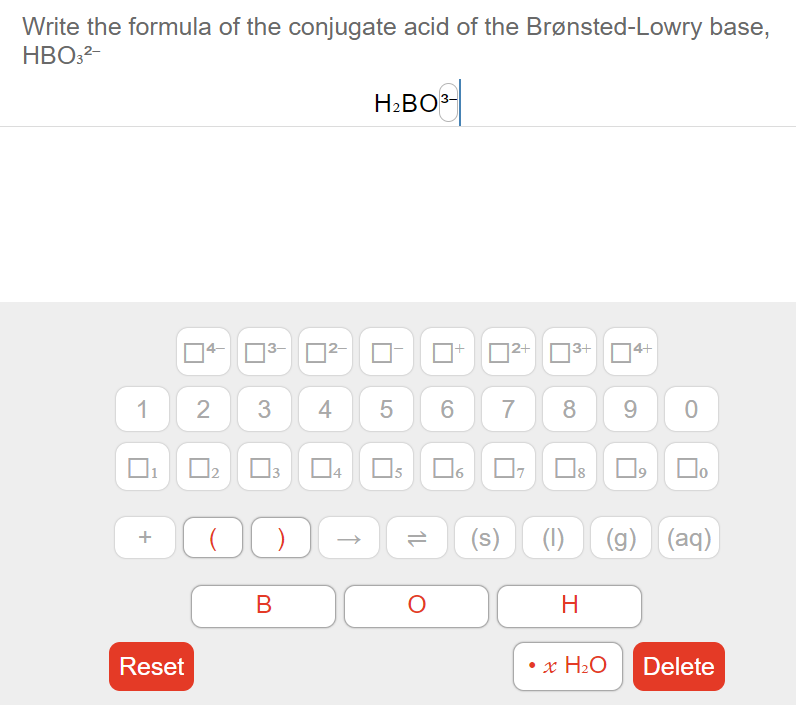
Answered Write The Formula Of The Conjugate Acid Bartleby
Web As we have found the pH we can now use the following formula to find the pOH.

. H2O l H2O l reversed arrows. What is the H3O and pH of pure water at body temperature. Web If the concentration given is OH- or H3O use the relation OH- x H3O 10-14 and solve for the unknown needed.
To find a concentration of hydronium ions. Web Calculating the Hydronium Ion Concentration from pH The hydronium ion concentration can be found from the pH by the reverse of the mathematical operation employed to find the. Web Lets calculate the pH of a strong acid solution.
Web The concentration of the hydrogen ion H is often used synonymously with the hydrated hydronium ion H 3 O. Web HNO3 aq and Ba OH2 aq Express your answer as a balanced chemical equation. H3O 10-14 269 x 10.
Calculate H 3 O and O H for each solution. Identify all of the phases in your answer. Web Answer- H3O 50E-5 Solution- pH is a negative logarithm of H3O.
For example in A. Enter the name of the chemical solution and its concentration value in the respective input fields. If wrong number of sig rigs on pH -1b.
In this case were gonna do a 0040M solution of nitric acid. Web H3O is extremely similar to H but they should not be considered identically the same thing. 2HNO3 aqBa OH2 aqBa NO32.
Calculate H3O for a solution of pH 43. Web H3Ofro water H3O from acidOH-10-14 Please note that H2O dissociates partially to form H3O and OH- and that this process reaches equilibrium. A 000165 M C H 3 cosH K a 1 8 10 5 b 00087 M K O H.
Web Again for simplicity H3O can be written as H in Equation 1663. Web At body temperature 37 degree C Kw 24 x 10-14. C 000213 M S r O H 2.
OH- Ph and PoH for pure water at 60 degrees C. 339 pOH 14 After subtracting 339 from both the pH and 14 we will get the pOH. HA aq H aq A aq Keep in mind though that free H does not exist in aqueous.
To find the OH- when H3O is known is to solve the above equation for OH-. H3O only is present in aqueous solutions while H ions can occur in. D 58 10 4 M H I.
Web The ionization constant for water kw is 9614x10-14 at 60 degrees C. Respond with the correct. Nitric acid is HNO3 and nitric acid reacts with water to form.
Web To use the pH calculator follow these steps.

12th Chemistry V2 Em 3 8 2019 10 13 Pdf Acid Dissociation Chemistry

Do Now Identify The Acids And Bases In The Following Equations Ppt Download

Calculate H O From Oh Youtube

16 1 Acids Bases In Water More Equilibrium Ppt Download

Topic 18 1 Calculations Involving Acids And Bases Ppt Download

Ppt Topic 18 Hl Acids Bases 18 1 Calcs Involving Acids And Bases Powerpoint Presentation Id 5405233

Ppt Topic 18 Hl Acids Bases 18 1 Calcs Involving Acids And Bases Powerpoint Presentation Id 5405233
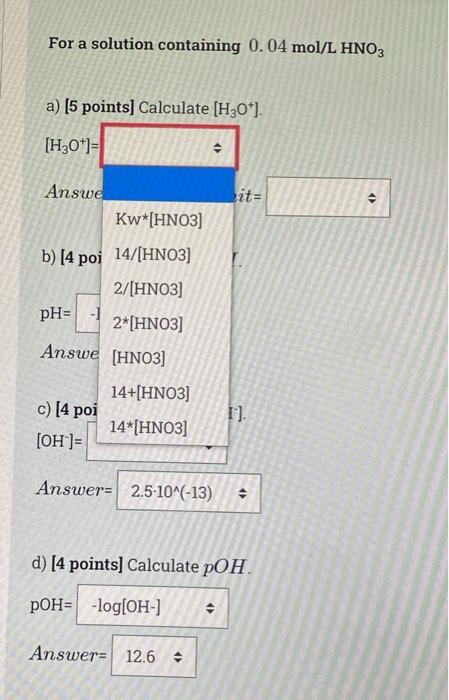
Solved For Each Part Of The Below Questions Choose One Chegg Com

Calculating The H Or H3o Using Ph Or Poh Youtube

The Molecular Nature Of Matter And Change Ppt Download

Calculated Depth Dose Distributions For H And He Beams In Liquid Water Request Pdf

Calculate H3o And Oh Using Kw Mcmurry Ch14 Problem 55 Youtube
How To Calculate H3o And Oh Sciencing
Solved Calculate Either H3o Or Oh For Each Of The Solutions Course Hero

Answered Fe Oh 3 H Ce Oh 4 H202 Ce3 Bartleby
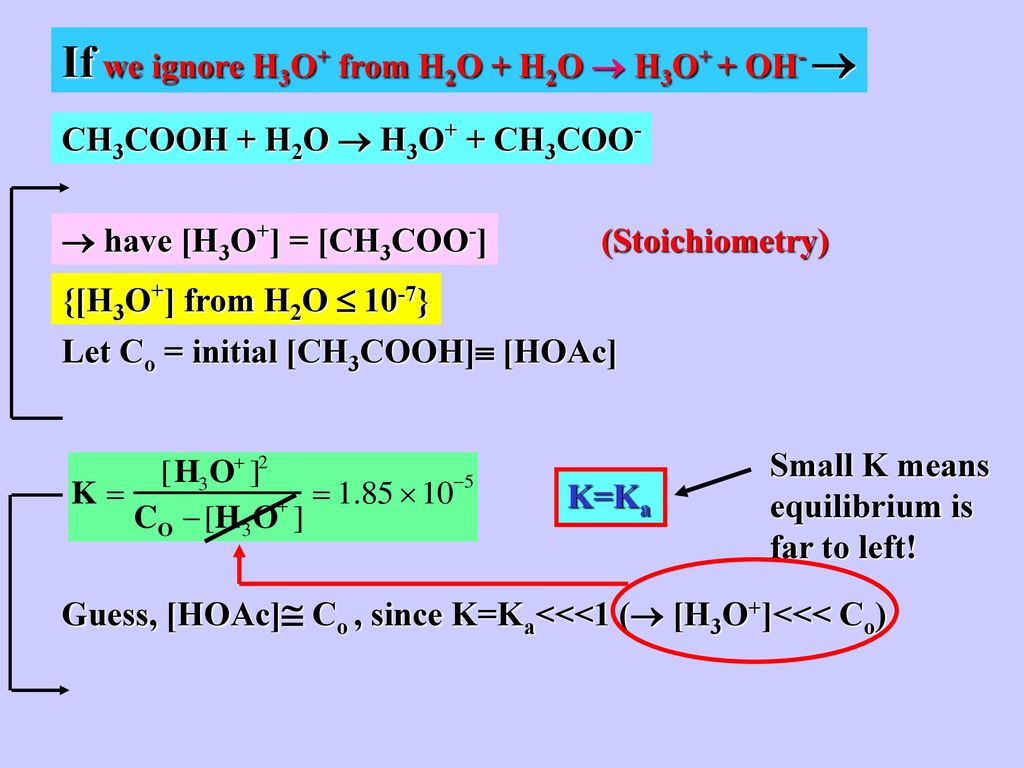
Suppose You Have H3o From Added Hcl 0 1 M 10 1 M Ppt Download
Solved Calculate Either H3o Or Oh For Each Of The Solutions Course Hero